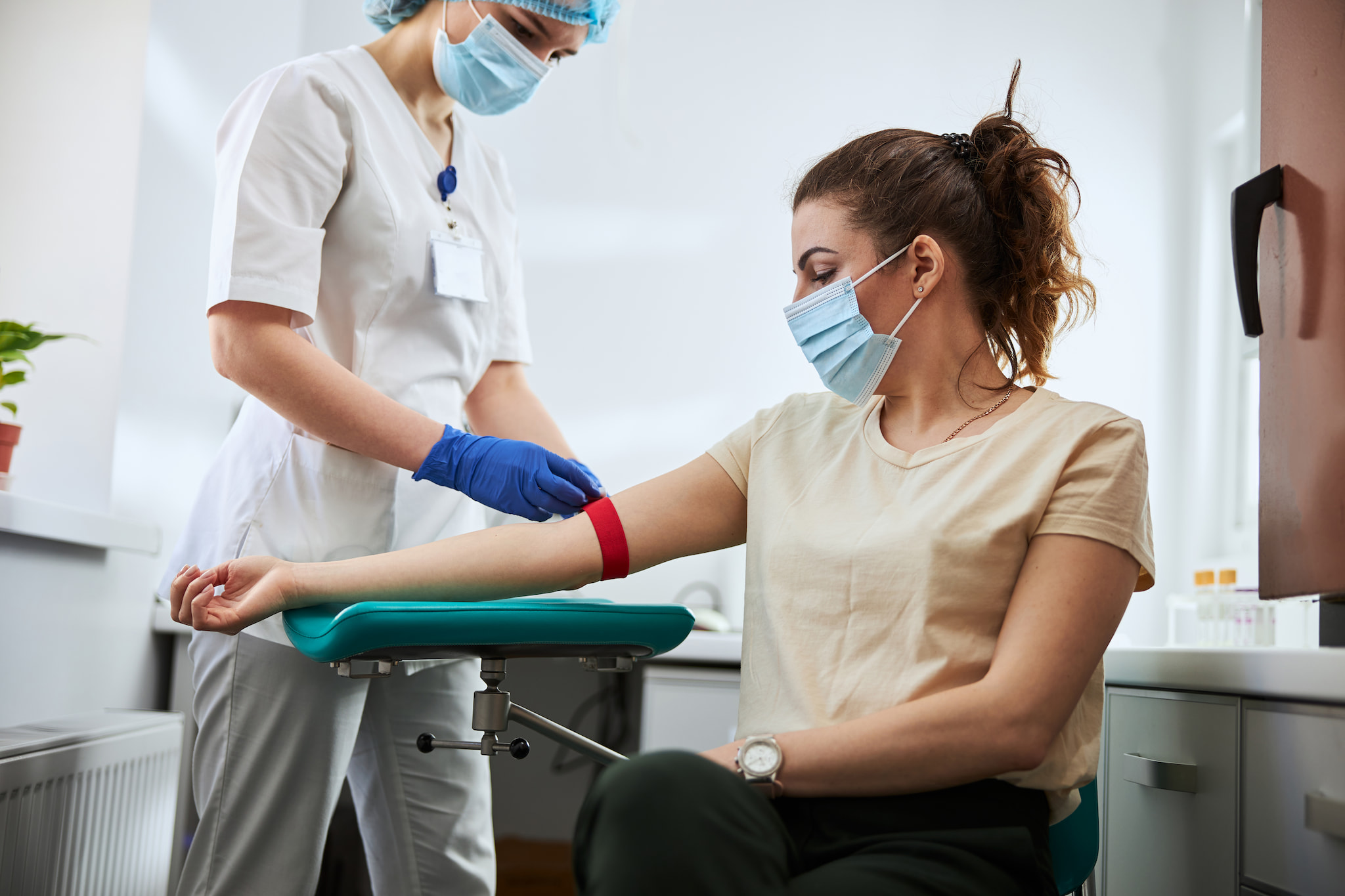
Biospecimen Procurement
Avrok is fully equipped to handle donor recruitment, consenting, sample collection, processing, and storage, all within one facility. We collect a wide range of sample types, including whole blood, tissue samples, leukopaks, and various other specimens. Emphasizing quality and high regulatory standards, Avrok is dedicated to meeting your research needs.
.png&w=2048&q=90)











CM%2C%20CGMBSTamanna%20Talukder%2C%20MS%2C%20MA%2C%20MB%20(ASCP)CM%2C%20CGMBS%20%C3%A2%C2%80%C2%A2%201st%20%C3%A2%C2%80%C2%A2%201stClinical%20Laboratory%20Scientist%20and%20Quality%20Assurance%20Manager%20at%20Avrok%20Bioscienc.jpg&w=2048&q=90)


.jpg&w=2048&q=90)
.png&w=2048&q=90)





